what is keto and enol form Enol form keto tautomers chemistry structures stable tautomerization forms which equilibrium between organic section tautomeric two differences bonding shown chemwiki
Enol forms are an important concept in organic chemistry, particularly in regards to their stability. In this post, we will explore the enol forms and discuss the stability of different enol forms. Additionally, we will examine keto-enol tautomerism, a fascinating phenomenon in organic chemistry. So, let’s dive in!
Enol Forms: Stability Discussion
Enol forms are a type of isomer that includes both an alkene (ene) and an alcohol (ol) functional group. These forms are most commonly found in compounds that contain a keto (carbonyl) group. The presence of a keto group allows for intramolecular hydrogen bonding, leading to the formation of the enol tautomer. It is important to note that the keto form is generally more stable than the enol form due to intramolecular hydrogen bonding.
 The image above illustrates a comparison between two enol forms, with one being more stable than the other. As we can see, the enol form on the left exhibits intramolecular hydrogen bonding between the hydroxyl group and the carbonyl group. This stabilizing interaction increases the stability of the enol form. On the other hand, the enol form on the right lacks such intramolecular hydrogen bonding, making it less stable than the counterpart.
The image above illustrates a comparison between two enol forms, with one being more stable than the other. As we can see, the enol form on the left exhibits intramolecular hydrogen bonding between the hydroxyl group and the carbonyl group. This stabilizing interaction increases the stability of the enol form. On the other hand, the enol form on the right lacks such intramolecular hydrogen bonding, making it less stable than the counterpart.
The stability of enol forms is influenced by various factors, including the availability of hydrogen bond acceptors and donors, steric hindrance, and resonance effects. By analyzing these factors, organic chemists can predict the relative stability of different enol forms and gain insights into their reactivity and behavior.
Keto-Enol Tautomerism
Keto-enol tautomerism refers to the interconversion between the keto and enol forms of a compound. This process occurs through the migration of a hydrogen atom and an adjacent double bond rearrangement. The equilibrium between the keto and enol forms is dynamic, with both forms existent but at varying concentrations. The ratio of these forms depends on factors such as temperature, solvent, and electronic effects.
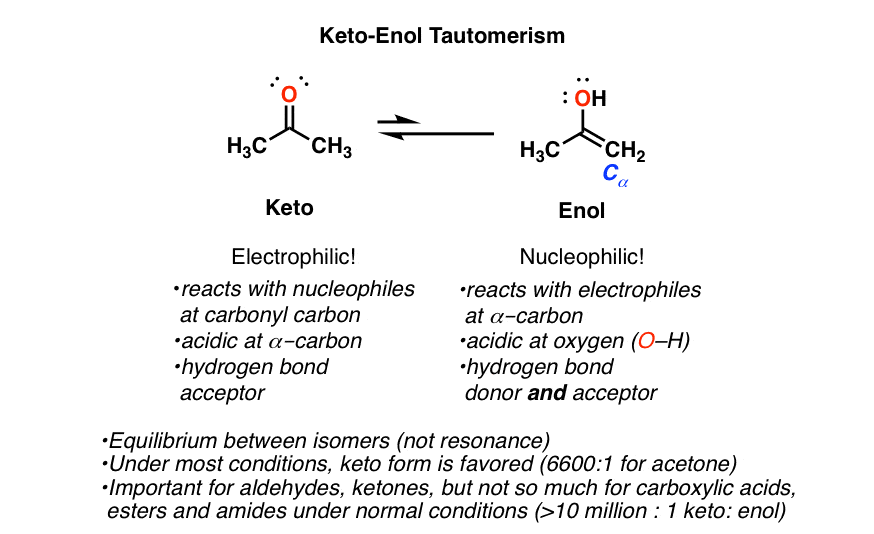 The image above highlights the equilibrium between the keto and enol forms in keto-enol tautomerism. In this case, the compound on the left exists predominantly in the keto form, while the compound on the right exists predominantly in the enol form. This equilibrium allows for dynamic interconversion, which plays a crucial role in various chemical reactions and processes.
The image above highlights the equilibrium between the keto and enol forms in keto-enol tautomerism. In this case, the compound on the left exists predominantly in the keto form, while the compound on the right exists predominantly in the enol form. This equilibrium allows for dynamic interconversion, which plays a crucial role in various chemical reactions and processes.
Keto-enol tautomerism has significant implications in organic chemistry, particularly in the synthesis of pharmaceuticals, natural products, and other complex organic molecules. By harnessing the interconversion between keto and enol forms, chemists can manipulate the reactivity and selectivity of reactions, leading to the desired product.
In conclusion, enol forms and keto-enol tautomerism are important concepts in organic chemistry. The stability of enol forms is influenced by factors such as intramolecular hydrogen bonding, steric hindrance, and resonance effects. Understanding the stability of enol forms helps predict their reactivity and behavior in chemical reactions. Keto-enol tautomerism, on the other hand, allows for dynamic interconversion between keto and enol forms, playing a crucial role in organic synthesis. By leveraging these concepts, organic chemists can explore new pathways and develop innovative approaches in their research and applications.
If you are searching about organic chemistry - Which is the more stable enol form? - Chemistry you’ve came to the right page. We have 5 Pics about organic chemistry - Which is the more stable enol form? - Chemistry like organic chemistry - Which is the more stable enol form? - Chemistry, Keto-Enol Tautomerism : Key Points - Master Organic Chemistry and also Keto Enol Tautomerization Reaction and Mechanism in Acid and Base. Read more:
Organic Chemistry - Which Is The More Stable Enol Form? - Chemistry
 chemistry.stackexchange.comenol form keto tautomers chemistry structures stable tautomerization forms which equilibrium between organic section tautomeric two differences bonding shown chemwiki
chemistry.stackexchange.comenol form keto tautomers chemistry structures stable tautomerization forms which equilibrium between organic section tautomeric two differences bonding shown chemwiki
Structural Isomerism - Organic Chemistry - Some Basic Principles And
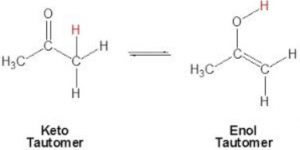 classnotes.org.inisomerism enol tautomerism triad type
classnotes.org.inisomerism enol tautomerism triad type
Keto-Enol Tautomerism : Key Points - Master Organic Chemistry
 www.masterorganicchemistry.comenol keto tautomerism base form points key chemistry reaction acid lewis draw each forms acetone but organic master
www.masterorganicchemistry.comenol keto tautomerism base form points key chemistry reaction acid lewis draw each forms acetone but organic master
Keto-Enol Tautomerism : Key Points - Master Organic Chemistry
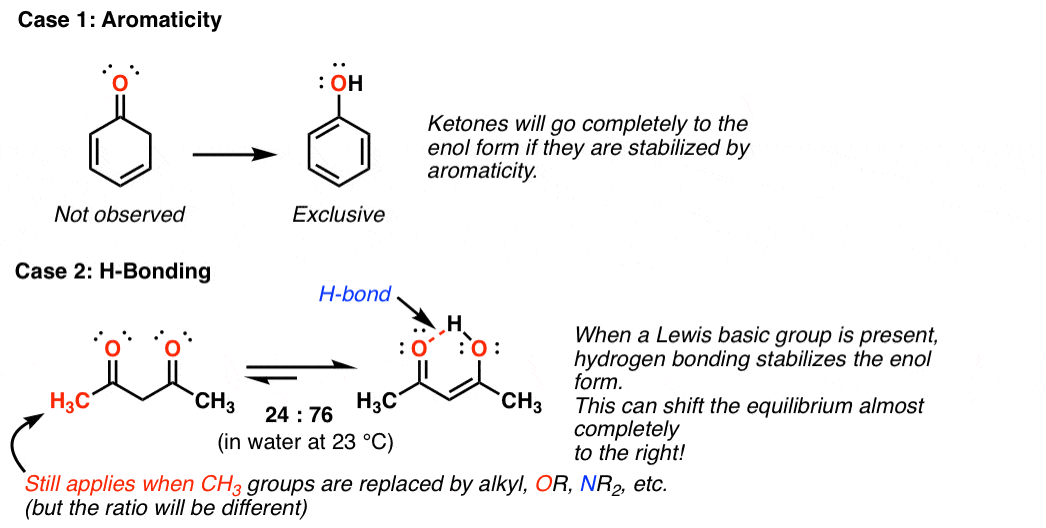 www.masterorganicchemistry.comenol keto tautomerism three points key form organic stabilized chemistry subtle effects
www.masterorganicchemistry.comenol keto tautomerism three points key form organic stabilized chemistry subtle effects
Keto Enol Tautomerization Reaction And Mechanism In Acid And Base
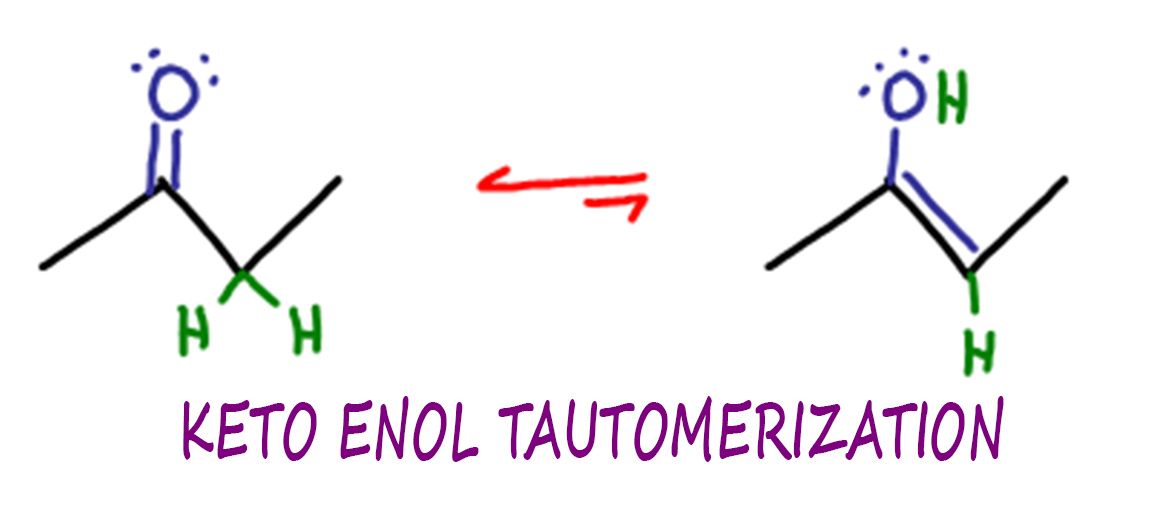 leah4sci.comenol tautomerization ketone catalyzed ket tautomers leah4sci molecules
leah4sci.comenol tautomerization ketone catalyzed ket tautomers leah4sci molecules
Isomerism enol tautomerism triad type. Enol keto tautomerism base form points key chemistry reaction acid lewis draw each forms acetone but organic master. Enol form keto tautomers chemistry structures stable tautomerization forms which equilibrium between organic section tautomeric two differences bonding shown chemwiki